世界の医療機器市場の規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
| 2024 –2031 | |
| USD 11.93 Billion | |
| USD 18.04 Billion | |
|
|
|
>世界の医療機器市場のセグメンテーション、製品別(人工呼吸器、スパイロメーター、酸素濃縮器、麻酔器、CPAP/BIPAP)、モード別(ポータブル、卓上、スタンドアロン)、用途別(診断および治療)、施設別(大規模、小規模、中規模)、エンドユーザー別(病院、外来手術センター、専門クリニック、長期ケアセンター、リハビリテーションセンター、在宅ケア施設)、流通チャネル別(直接販売およびサードパーティ販売業者)– 2031年までの業界動向と予測
医療機器市場分析
医療機器市場は、技術の急速な進歩、医療ニーズの高まり、高齢化の進行により、大幅な成長を遂げています。遠隔医療、ウェアラブル健康モニタリング機器、低侵襲手術器具などのイノベーションは、市場環境を一変させ、患者ケアを強化し、臨床結果を改善しています。たとえば、診断用画像機器に人工知能(AI)と機械学習を統合することで、医療提供者はより正確な診断と個別化された治療計画を実現できます。さらに、COVID-19パンデミックにより、遠隔医療サービスと遠隔患者モニタリング機器の導入が加速し、市場拡大がさらに加速しています。企業は、慢性疾患の管理やリハビリテーションなど、特定の健康上の課題に対処する革新的なソリューションを生み出すために、研究開発に投資しています。慢性疾患の増加と在宅医療ソリューションの需要も、市場成長の要因となっています。規制当局は、新しい医療機器の承認プロセスをますます合理化しており、画期的な製品の市場参入を早めています。その結果、医療機器市場は大幅に拡大し、メーカー、医療提供者、患者にチャンスがもたらされることになります。
医療機器市場規模
世界の医療機器市場規模は、2023年に119億3,000万米ドルと評価され、2024年から2031年の予測期間中に5.30%のCAGRで成長し、2031年には180億4,000万米ドルに達すると予測されています。市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、Data Bridge Market Researchがまとめた市場レポートには、詳細な専門家分析、患者の疫学、パイプライン分析、価格分析、規制枠組みも含まれています。
医療機器市場の動向
「遠隔医療技術の統合の拡大」
One significant trend shaping the medical devices market is the increasing integration of telehealth technologies, which has gained tremendous momentum, especially in the wake of the COVID-19 pandemic. Telehealth devices, including remote patient monitoring systems and mobile health applications, allow healthcare professionals to monitor patients' health from a distance, improving access to care and reducing the burden on healthcare facilities. For instance, wearable devices and smartwatches with health-tracking capabilities are becoming popular, enabling users to monitor vital signs such as heart rate and oxygen levels in real time. This trend enhances patient engagement and facilitates timely interventions, particularly for chronic disease management. Moreover, healthcare providers are leveraging telehealth solutions to conduct virtual consultations, thus expanding their reach to underserved populations. As a result, the telehealth trend is driving innovation within the medical devices market, prompting manufacturers to develop advanced solutions that prioritize connectivity and user-friendliness while ensuring compliance with regulatory standards.
Report Scope and Medical Devices Market Segmentation
|
Attributes |
Medical Devices Key Market Insights |
|
Segments Covered |
|
|
Countries Covered |
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America |
|
Key Market Players |
GE Healthcare (U.S.), Koninklijke Philips N.V. (Netherlands), Medtronic (U.S.), Drägerwerk AG & Co. KGaA (Germany), VYAIRE (U.S.), Getinge AB (Sweden), NDD Medical Technologies (Switzerland), ResMed (U.S.), Invacare Corporation (U.S.), NIDEK MEDICAL (Japan), O2 CONCEPTS, LLC (U.S.), Teijin Limited (Japan), GCE Healthcare (U.K.), Inogen, Inc (U.S.), Teleflex Incorporated (U.S.), Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (China), MGC Diagnostics Corporation (U.S.), HILL-ROM (U.S.), Drive DeVilbiss Healthcare Inc. (U.S.), Midmark Corporation (U.S.), CAIRE Inc. (U.S.), GCE Group (U.K.), Fisher & Paykel Healthcare Limited (New Zealand), and Schiller (Switzerland) |
|
Market Opportunities |
|
|
Value Added Data Infosets |
In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework. |
Medical Devices Market Definition
Medical devices are instruments, apparatus, machines, or implants that are designed for medical purposes, including diagnosis, prevention, monitoring, treatment, or alleviation of diseases and medical conditions. These devices range from simple tools and bandages and thermometers to complex technologies such as imaging systems, surgical instruments, and implantable devices and pacemakers. They play a crucial role in modern healthcare by enhancing patient care, improving outcomes, and enabling healthcare professionals to deliver accurate and efficient treatments.
Medical Devices Market Dynamics
Drivers
- Rising Healthcare Expenditure
Rising healthcare expenditure is a significant driver of the medical devices market, as countries worldwide allocate more resources to enhance their healthcare systems. For instance, according to the World Health Organization (WHO), global health spending is projected to rise by 6% annually, significantly outpacing economic growth. In the United States, healthcare expenditure reached approximately USD 4.1 trillion in 2020, accounting for nearly 20% of the GDP. This surge in investment facilitates the acquisition of advanced medical devices, enabling healthcare providers to adopt innovative technologies that improve patient outcomes and streamline operations. For instance, hospitals are increasingly integrating advanced imaging systems and robotic surgical instruments into their practices to enhance diagnostic capabilities and surgical precision. As a result, the escalating focus on healthcare spending is creating robust demand for cutting-edge medical devices, propelling market growth.
- Increase in Chronic Diseases
The increase in chronic diseases is a significant driver of the medical devices market, as the growing prevalence of conditions such as diabetes, cardiovascular diseases, and respiratory disorders creates a heightened demand for advanced diagnostic and management solutions. According to the World Health Organization (WHO), chronic diseases are responsible for approximately 71% of global deaths each year, with cardiovascular diseases alone claiming around 17.9 million lives annually. For instance, the International Diabetes Federation estimates that approximately 537 million adults were living with diabetes in 2021, a number projected to rise to 643 million by 2030. This surge necessitates the deployment of innovative medical devices, such as continuous glucose monitors, advanced imaging technologies, and telehealth solutions, which are crucial for effective disease management and monitoring. As healthcare providers seek to improve patient outcomes and manage these chronic conditions more effectively, the demand for sophisticated medical devices is expected to grow substantially, thereby driving market expansion.
Opportunities
- Increasing Technological Advancements in Medical Devices
Technological advancements present a significant market opportunity in the medical devices sector, particularly with innovations such as minimally invasive surgical devices, wearable health monitors, and telehealth solutions. These advancements are transforming the landscape of patient care by enhancing the effectiveness and appeal of medical devices. For instance, the development of robotic-assisted surgical systems has enabled surgeons to perform complex procedures with greater precision and reduced recovery times, improving patient outcomes and satisfaction. Similarly, wearable health monitors, and smartwatches equipped with heart rate and activity tracking, are empowering consumers to take control of their health by providing real-time data on vital signs and encouraging proactive health management. Moreover, the rise of telehealth solutions, accelerated by the COVID-19 pandemic, has expanded access to healthcare services, allowing patients to consult with healthcare providers remotely. This growing trend underscores the increasing demand for innovative medical devices that facilitate remote monitoring and diagnostics, creating a lucrative opportunity for manufacturers and investors in the medical devices market.
- Regulatory Support from Governments and Regulatory Bodies
Regulatory support from governments and regulatory bodies is emerging as a significant market opportunity in the medical devices sector, as it streamlines the development and approval processes for new innovations. Initiatives such as the FDA’s Breakthrough Devices Program in the U.S. aim to expedite the review of devices that provide more effective treatment options for life-threatening or irreversibly debilitating diseases. For instance, this program has helped bring cutting-edge technologies and advanced cardiac monitoring devices and novel insulin delivery systems to market more quickly, ultimately benefiting patients with critical healthcare needs. Similarly, the European Union’s Medical Device Regulation (MDR) encourages manufacturers to enhance product safety and performance, thereby fostering trust in new technologies. This regulatory environment facilitates faster market entry and encourages manufacturers to invest in research and development, driving innovation in the sector. As a result, companies that effectively navigate these supportive regulatory frameworks are better positioned to capitalize on emerging market trends and fulfill the growing demand for advanced medical devices.
Restraints/Challenges
- High Development Costs
High development costs represent a significant challenge in the medical devices market, often acting as a barrier to entry for new companies. The research and development (R&D) phase necessitates a substantial investment in technology, resources, and expertise. This investment includes conducting extensive clinical trials and testing protocols to ensure that devices meet safety and efficacy standards set by regulatory bodies. For instance, the development of innovative devices such as implantable cardiac monitors can cost millions of dollars, which may deter start-ups and smaller firms that lack the financial resources compared to established companies. In price-sensitive markets, these high costs can further limit the scope for innovation, as companies may prioritize developing lower-cost products rather than pursuing ground-breaking technologies. This financial burden can ultimately hinder market diversity and the introduction of novel medical solutions, stifling potential advancements in patient care.
- Patient Privacy Concerns
Patient privacy concerns are increasingly significant challenges in the medical devices market, particularly with the growing adoption of digital health technologies. As devices become more interconnected and reliant on cloud-based data storage, the risk of unauthorized access to sensitive patient information escalates. For instance, wearable health monitors that track vital signs and transmit data to healthcare providers must ensure robust encryption and security measures to protect patient data. A breach in data security jeopardizes patient trust but can also lead to severe regulatory penalties and reputational damage for manufacturers. Healthcare providers are hesitant to adopt technologies that do not prioritize patient privacy, which can hinder the widespread acceptance of innovative medical devices. Consequently, manufacturers must invest in developing secure solutions that comply with regulations such as the Health Insurance Portability and Accountability Act (HIPAA) to ensure data protection and build trust among consumers and healthcare professionals.
This market report provides details of new recent developments, trade regulations, import-export analysis, production analysis, value chain optimization, market share, impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, strategic market growth analysis, market size, category market growths, application niches and dominance, product approvals, product launches, geographic expansions, technological innovations in the market. To gain more info on the market contact Data Bridge Market Research for an Analyst Brief, our team will help you take an informed market decision to achieve market growth.
Medical Devices Market Scope
The market is segmented on the basis of product, mode, application, facility, end user, and distribution channel. The growth amongst these segments will help you analyse meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Product
- Ventilator
- Spirometers
- Oxygen Concentrators
- Anesthesia Machines
- CPAP/BIPAP
Mode
- Portable
- Tabletop
- Standalone
Application
- Diagnostic
- Therapeutic
Facility
- Large
- Small and Medium
End User
- Hospital
- Ambulatory Surgical Centres
- Specialty Clinics
- Long Term Care Centers
- Rehabilitation Centers
- Homecare Settings
Distribution Channel:
- Direct Sales
- Third Party Distributor
Medical Devices Market Regional Analysis
The market is analysed and market size insights and trends are provided by country, product, mode, application, facility, end user, and distribution channel as referenced above.
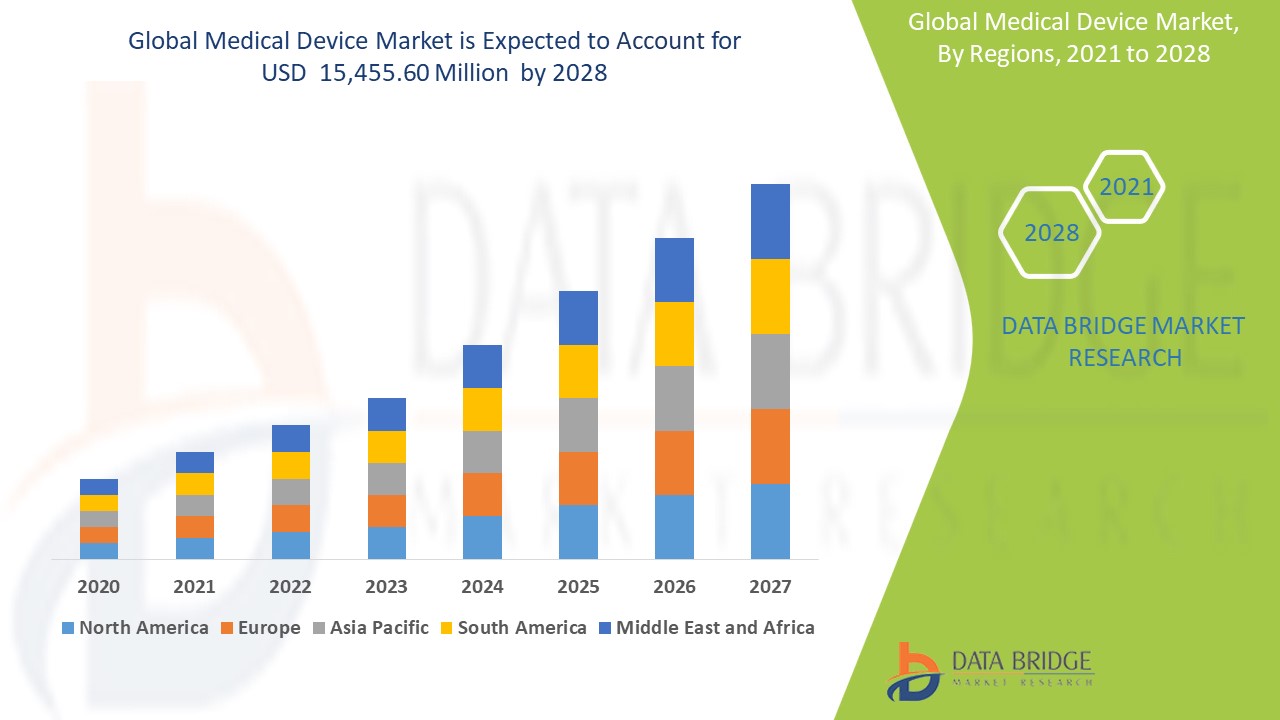
The countries covered in the market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E., South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
North America leads the Medical Devices market, driven by its advanced healthcare infrastructure and a rising prevalence of chronic diseases that necessitate sophisticated diagnostic tools. The region also benefits from substantial investments and funding aimed at enhancing medical technology, which fosters innovation in microscopy. Moreover, the presence of major industry players contributes to a competitive landscape that stimulates further advancements. Additionally, growing research and development efforts in various scientific fields are propelling the demand for high-quality video microscopy solutions in North America.
Asia-Pacific is projected to experience significant growth from 2024 to 2031, fueled by increasing investments in research and development that enhance healthcare capabilities. The rising geriatric population in the area is leading to higher demand for effective diagnostic and treatment options. Furthermore, the escalating incidence of chronic diseases drives the need for advanced medical products and technologies. Additionally, the growing acceptance and adoption of innovative medical solutions in this region are set to bolster market expansion during the forecast period.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points such as down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Medical Devices Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
医療機器市場で活動するマーケットリーダーは次のとおりです。
- GEヘルスケア(米国)
- Koninklijke Philips NV (オランダ)
- メドトロニック(米国)
- Drägerwerk AG & Co. KGaA (ドイツ)
- VYAIRE(米国)
- ゲティンゲ AB (スウェーデン)
- NDDメディカルテクノロジーズ(スイス)
- レスメド(米国)
- インバケアコーポレーション(米国)
- ニデックメディカル(日本)
- O2 CONCEPTS, LLC(米国)
- 帝人株式会社(日本)
- GCEヘルスケア(英国)
- Inogen, Inc (米国)
- テレフレックス株式会社(米国)
- 深センミンレイバイオメディカルエレクトロニクス株式会社(中国)
- MGCダイアグノスティクスコーポレーション(米国)
- ヒルロム(米国)
- ドライブデビルビスヘルスケア社(米国)
- ミッドマークコーポレーション(米国)
- CAIRE Inc.(米国)
- GCEグループ(英国)
- フィッシャー&パイケルヘルスケアリミテッド(ニュージーランド)
- シラー(スイス)
医療機器市場の最新動向
- 2024年9月、英国を拠点とする人間工学に基づいた顕微鏡と測定システムの設計・製造会社であるビジョンエンジニアリングは、特許取得済みの光学実体顕微鏡技術のエントリーレベル製品であるOPTAの世界発売を発表しました。
- 2024年8月、ヴィジョン・エンジニアリングは、特許取得済みの光学実体顕微鏡技術の新たなエントリーポイントとして、わずか1,167米ドルで購入可能なOPTAを世界的に発売しました。この製品は、3つのスタンドと2つのレンズの選択肢を提供し、優れた画質、人間工学に基づいたデザイン、使いやすさを求めるユーザーにとって新たな基準を確立しています。
- 2024年7月、革新的なヘルスケアソリューションを専門とする医療機器会社Medprime Technologiesは、インドのデジタル病理学を変革することを目的とした画期的なAI統合デジタル顕微鏡プラットフォームMicalysを発表しました。
- 2021年7月、カールツァイスは、ZEISS Xradia 3D X線プラットフォームの一部として、ZEISS PhaseEvolveとZEISS DeepRecon Pro再構成技術を発表しました。これらの高度な技術は、人工知能(AI)を活用してデータの収集と分析を強化し、意思決定プロセスを迅速化し、業界の成長を促進します。
- 2021年6月、ダナハーコーポレーションは、実験の成功率を高め、光学顕微鏡と電子顕微鏡(EM)の統合を簡素化するように設計された革新的な生細胞相関光学電子顕微鏡(CLEM)ソリューションであるライカナノワークフローを発表しました。この高度なワークフローは、効率的で正確なイメージング技術に対する高まる需要に応え、研究者が生細胞内の生物学的プロセスを、より高い精度と使いやすさで研究できるようにします。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。